Australian regenerative medicine company and creator of allogeneic cellular drugs for complex inflammatory diseases, Mesoblast [ASX:MSB] has now said it has narrowed its losses taken after tax from US$21.3 million last year to US$18.6 million — a US$2.7 million improvement from 2022.
The group also announced its Remestemcel-L BLA filing was accepted by the US Food and Drug Administration (FDA), with a new Prescription Drug User Fee Act (PDUFA) goal date set and ongoing manufacturing inspections.
Revenue from royalties on sales of Temcell sold in Japan grew 4% in the period to $US2 million for the quarter, and a US$40 million placement has also upped funds.
Year-to-date, MSB has risen nearly 25% in share price and more than 9% in the last month alone.
Right now, it is trending above its sector by 2.5% and more than 11% in the S&P 200:
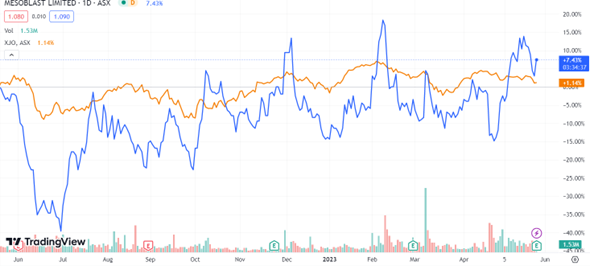
Source: tradingview.com
Mesoblast cuts down its year-on-year losses
Cell technology research and medicines developer Mesoblast has announced that it has trimmed losses after tax from US$21.3 million in the March quarter last year to US$18.6 million this most recent quarter.
The announcement was given as an operational highlights and financial results report for the period ending 31 March 2023.
Net cash usage for operating activities in the quarter reached US$16.2 million, representing an increase of US$0.7 million, or 4%, on the comparative quarter in FY2022 and a reduction of US$8.3 million, or 34%, on the comparative quarter in FY2021.
Research and Development expenses were reduced by US$1.2 million (14%), down to US$7.0 million for the third quarter of FY2023 compared to US$8.2 million for the third quarter of FY2022.
Manufacturing expenses were slightly higher than in 2022, having climbed from US$5.6 million in the third quarter of 2022 to US$6.2 million this past quarter.
In terms of income, revenue from royalties on sales of Temcell sold in Japan grew by 4% during this time to US$2 million for the quarter.
This was compared with US$1.9 million for the quarter that ended 31 March 2022.
The biotech explained that the US FDA has now accepted its remestemcel-L in the treatment of children with steroid-refractory graft versus host disease (SR-aGVHD) and has set the goal date for a PDUFA for 2 August.
The FDA has also now conducted the Pre-License Inspection (PLI) of the manufacturing process for remestemcel-L, and an Establishment Inspection Report (EIR) is expected to be issued by FDA in the coming weeks to provide a detailed summary and final assessment.
The company’s Regenerative Medicine Advanced Therapy (RMAT) has also been granted approval by the FDA, clearing a trial protocol and is expected to commence enrolment for these trials by the third quarter.
MSB has successfully completed a global private placement primarily for existing major US, UK, and Australian shareholders, which has raised around US$40 million, net of transaction costs.
The group finished the quarter with US$48.8 million cash on hand pro-forma cash after adjusting for US$40.0 million of proceeds raised in April is US$88.8 million, with up to an additional US$40.0 million available to be drawn down from existing financing facilities subject to certain milestones.
Tik-stocks and the mania of 2024
Did you know that previous no-name and inconspicuous small-cap Stemcell United [ASX:SCU] catapulted 8,284% in two days on a medicinal cannabis venture?
It wasn’t so insignificant then, and it happened so suddenly.
Cann Group [ASX:CAN], too, jumped by 30 cents to $4 in a manner of months on another viral explosion.
If you know where the next burst of mania is going to be, you can really take advantage of the hype.
This is why our experts bring you Tik-Stocks, a sort of cousin to ‘meme stocks’ — you know, those stocks that go through the roof based on social media coverage.
These stocks can become overvalued in the blink of an eye. This means you need to time them right.
Intrigued? Click here for more.
Regards,
Mahlia Stewart,
For Money Morning

