The biopharmaceutical discovery and development company, Adalta Ltd [ASX:1AD] share price is higher today, after announcing its product had received Orphan Drug Designation from the US Food and Drug Administration.
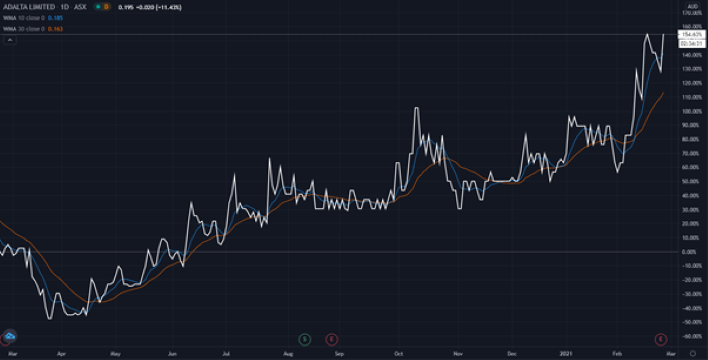
At time of writing the 1AD share price is up 2 cents, or 11.43%, to trade at 19.5 cents per share.
The 1AD share price has had a great run over the past 12 months, with the pandemic putting the wind in the sails of many stocks within the ASX healthcare sector.
Stay up to date with the latest investment trends and opportunities. Click here to learn more.
Adalta takes inspiration from sharks to save people
1AD announced today that its lead product candidate AD-214 (used in the treatment of Idiopathic Pulmonary Fibrosis) has received orphan status from the FDA.
The biopharmaceutical developer is using its proprietary technology platform to generate a new class of single domain antibody protein therapeutics, known as i-bodies.
The technology mimics the shape and stability of an antigen-binding domain, that was discovered initially in sharks and then developed as a human protein.
Orphan status 1AD received today entitles AD-214 to certain benefits during development including eligibility for seven years market exclusivity post approval, tax credits, reduced review times, and the waiver of certain marketing authorisation application fees.
Among other things.
The status is given to potential treatments for rare or ‘orphan’ diseases that affect fewer than 200,000 people in the US.
Their treatment’s appointment to orphan status comes after encouraging results from their Phase 1 clinical study.
1AD said that the study also showed higher expected receptor occupancy seven days after dosing.
Which they say suggests potential for longer duration of effect and therapeutic dosing interval.
Meaning the positive effects of the treatment last longer than the company anticipated.
What’s next for Adalta Share Price?
The final results from Phase 1 Part A are expected in April with Part B of the clinical studies on track to start in the June 2021 quarter.
Part B will trial the treatment on patients with Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease.
Should the final results of Part A be in line with what we have currently seen, I would anticipate the 1AD share price to follow a similar trajectory to what we are currently seeing.
The reason I say that is because now that 1AD has a big partner in that of GE Healthcare, that should help identify additional indications for AD-214 and the selection of the next targets for their i-body platform.
Regards,
Lachlann Tierney,
For Money Morning
PS: If you think using shark antibodies to treat disease is innovative, then check out these four well-positioned small-cap stocks — these innovative Aussie companies are well placed to capitalise on post-lockdown megatrends. Click here to learn more.