Allogeneic cellular medicine developer focused on treating severe and life-threatening inflammatory conditions Mesoblast [ASX:MSB] has experienced a 10.5% boost in share price after providing an update on its Remestemcel-L product for children with SR-aGVHD (Steroid-Refractory Active Graft Vs Host Disease).
Mesoblast had several updates to share today, including the resubmission of its Biologic Licence Application (BLA) to the FDA, a validation of potency and activity efforts for Phase 3 trials, and new data proving Remestemcel-L improved inflammatory biomarkers and survival in high-risk cases.
MSB’s share price has bumped up 20% in the last month, however, it slid well over 10% over the last year from early January 2022 to now.
An MSB share is worth $1.05 at time of writing.
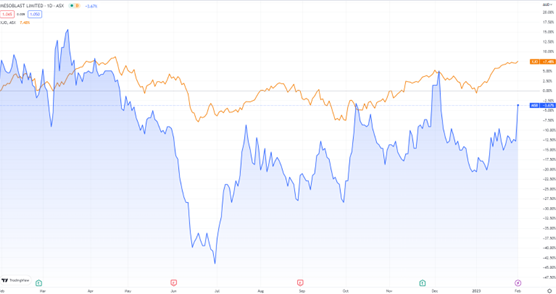
Source: TradingView
Mesoblast bolsters investors with positive Remestemcel-L updates
The allogeneic medicine maker today said that it’s resubmitted its Biologics License Application (BLA) to the US Food and Drug Administration (FDA), seeking approval for use of its Remestemcel-L in the treatment of children with SR-aGVHD.
Following guidance from the FDA, Mesoblast generated new data and analysis for the resubmission of its product licence, data which it believes displays significant evidence of Remestemcel-L’s effectiveness in children with the life-threatening disease.
New data shows long-term survival for children in Phase 3 of the trial, supporting durability for treatment through to a maximum of four years, and shows benefits as a treatment for high-risk cases according to validated biomarkers.
Mesoblast also noted increased potency changes had a positive effect on survival rates, a formula that the company will use to improve future commercial batches of Remestemcel-L to boost survival outcomes.
Chief Executive of Mesoblast Dr Silviu Itescu stated:
‘There is an urgent need for a therapy that improves the dismal survival outcome in children with SR-aGVHD.
‘Our team has worked tirelessly over the past two years to provide a comprehensive response to the FDA. We are grateful for the agency’s active dialogue and constructive feedback that will ensure a high bar is met in terms of product consistency and predictability of clinical outcomes.’
Hard facts and breakthroughs for biotech
The company warned that outcomes over the past 20 years have not improved for those suffering from the most severe forms of SR-aGVHD.
The lack of approved treatments for children under 12 translates to an urgency for therapy and a massive hole in the market that needs addressing.
MSB believes that if it receives that FDA approval for Remestemcel-L, it’ll be the very first of its kind, the first ‘off-the-shelf’ medicine to be offered in the US, and first-at-hand therapy for children with the disease.
Yesterday, the medicinal pioneer reported financial highlights for the quarter ended 31 December 2022.
Revenue from royalty sales of TEMCELL in Japan increased 36% to US$1.9 million, while operational net cash reduced $1.7 million in the quarter.
Cash on hand at the end of the quarter was US$67.6 million, with up to an additional US$40 million available to be drawn down from existing financing facilities subject to certain milestones.
Incoming! Commodity boom Australia
Our resources expert and trained geologist, James Cooper, thinks the Australian resources sector is set to enter a new commodities boom brought on by the ‘Age of Scarcity’.
Similar patterns that occurred 20 years ago are happening again.
James is convinced ‘the gears are in motion for another multi-year boom in commodities’.
A boom where Australia (and ASX stocks) stands to benefit…
The next big mining boom is predicted to happen in the next few years.
The same investors that got rich last time are preparing to make their move — don’t let them take the monopoly again.
You can learn from James’ experiences AND access an exclusive video on his personalised ‘attack plan’ right here.
If that isn’t enough to sate your curiosity, check out last year’s interview with James and Greg with Ausbiz.
Regards,
Mahlia Stewart,
For The Daily Reckoning Australia

