The Immutep Ltd [ASX:IMM] share price is up 11.4% today after securing fast track designation from the US FDA for its eftilagimod alpha product.
The biotechnology company, which develops immunotherapy treatments for cancer, infectious disease and autoimmune disease, stated the fast track designation can expedite development and review with the US FDA.
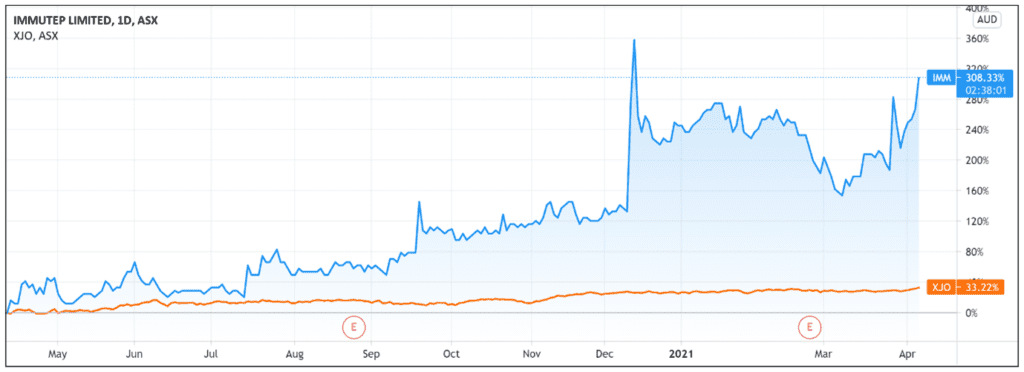
IMM shares were up as much as 17% in early trade.
After falling in early March, IMM shares are currently up 55% over the last month and up 250% over the last 12 months.
Immutep’s efti product achieves fast track designation
Immutep revealed that its lead product candidate — eftilagimod alpha (efti), a soluble LAG-3 protein — received the fast track designation in first line recurrent or metastatic HNSCC from the United States Food and Drug Administration (FDA).
We reveal four little-known small-cap stocks that cannot be ignored…Download your free report now.
Head and neck squamous cell carcinoma (HNSCC) develop in the mucous membranes of the mouth, nose and throat.
Squamous cells are found in the outer layer of the skin in the mucous membranes.
Immutep stated that the fast track was granted because of efti’s ‘potential to address an unmet medical need, as evidenced by encouraging data indicating a positive risk benefit ratio.’
Immutep noted that the data package evaluated by the FDA ‘included promising results’ from Part C of Immutep’s Phase II TACTI-002 trial assessing efti in combination with KEYTRUDA (pembrolizumab).
As the company explained in the release, an FDA fast track designation is awarded to help ‘important new therapies reach patients faster.’
Immutep secures European patent grant
Yesterday, Immutep announced that the European Patent Office granted the company a patent for one of its antibody products.
As the company explained, the claims of the patent are directed to embodiments of LAG525, a humanised form of Immutep’s IMP701 antibody, out-licensed to Novartis AG.
IMP701 is a therapeutic antibody that can ‘remove two brakes that prevent the immune system from responding to and killing cancer cells.’
The patent is co-owned by Novartis AG and Immutep and expires on 28 July 2036.
Immutep share price outlook
Immutep noted in today’s announcement that its new Phase IIb trial in first line HNSCC, TACTI-003 to evaluate efti in combination with KEYTRUDA is expected to start in mid-2021.
The trial will run in collaboration with MSD, a subsidiary of Merck & Co.
Additionally, the company’s humanised form of IMP701 is currently being evaluated by Novartis in several Phase 1 and Phase 2 clinical trials.
The bidding up of IMM shares this week suggests investors are bullish on Immutep’s progress.
In a sector so contingent on regulatory approvals, investors may have seen the FDA fast tracking one of Immutep’s products as a positive indication the company is progressing.
However, as with any junior biotechnology stock, many more hurdles are coming and Immutep will have to scale each one successfully to commercialise its products.
No doubt investors will be eagerly watching Immutep’s upcoming clinical results.
If you want further coverage and analysis of biotechnology stocks and related investment ideas, then I suggest checking out our free, daily e-letter.
It’s a publication that brings you investing news you’ll be hard-pressed to find anywhere else.
Regards,
Lachlann Tierney,
For Money Morning