The AnteoTech Ltd’s [ASX:ADO] share price is up 17% at time of writing after a clinical study shows AnteoTech’s COVID-19 test can meet WHO’s guidelines.
In today’s ASX announcement, nanotechnology company AnteoTech reported the outcome of its COVID-19 Antigen Rapid Test (ART) clinical laboratory validation study.
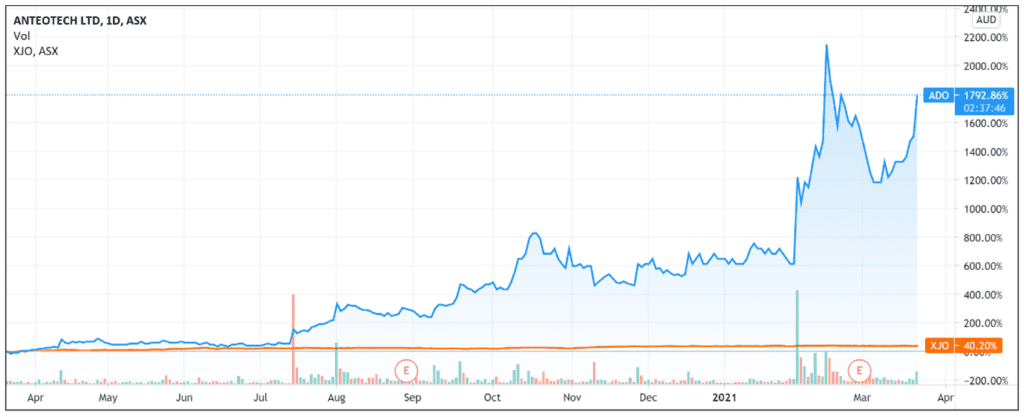
The market received AnteoTech’s ASX update well, with ADO shares up 17.7% at 1:45pm.
The market’s response to AnteoTech’s announcement reflects the strong investor support enjoyed by the company in recent months.
Year-to-date, the AnteoTech share price is up 152% and up more than 1,700% in more than a year.
AnteoTech clinical study results
According to the company, AnteoTech’s Antigen Rapid Test detects the SARS-CoV-2 active virus that causes COVID-19.
Stay up to date with the latest investment trends and opportunities. Click here to learn more.
As a recent WHO report noted, rapid antigen detecting tests ‘may facilitate earlier diagnosis and required actions … in settings where RT-PCR is unavailable or turnaround times for results are slow.’
Overall, the study showed that AnteoTech’s COVID-19 ART sensitivity was 97.3% (179/184).
Out of 184 de-identified nasopharyngeal samples clinically diagnosed as positive for SARS-CoV-2, AnteoTech’s ART correctly detected 179 out of the 184 of them as positive.
The COVID-19 ART sensitivity was 99.6% (259/260).
Out of 260 samples clinically diagnosed as negative, AnteoTech’s COVID-19 ART correctly classified 259 of them as negative.
The study was conducted and reviewed by the Victorian Infectious Diseases Reference Laboratory (VIDRL), which is part of the Peter Doherty Institute for Infection and Immunity.
The Peter Doherty Institute led Australia’s research efforts when the virus first broke out and was the first to isolate and share the virus outside of China.
What is the significance of AnteoTech’s ASX announcement?
AnteoTech’s laboratory validation study was conducted to ‘demonstrate that the test meets the World Health Organisation’s recommended guidelines for COVID-19 antigen rapid tests.’
Why is this important?
Meeting WHO’s recommended guidelines will go a long way in securing a CE mark.
A CE mark indicates that a product was assessed by the manufacturer and met EU safety, health, and environmental protection requirements.
A CE mark is required for many products before they can be sold in the European Union.
Commenting on the clinical study results, AnteoTech CEO Derek Thomson said:
‘We are delighted to have achieved this significant milestone in the development of AnteoTech’s first COVID-19 ART.
‘The study enables us to be compliant with WHO guidelines for market use of our COVID-19 antigen rapid test and provides the data required for use in our CE regulatory submission.’
ADO share price outlook
According to the World Health Organisation, there were more than 700 products released onto the market to detect SARS-CoV-2-specific nucleic acids, antigens, and human antibodies as of September 2020.
The space is crowded and not every product out of the more than 700 products out there will achieve market traction.
As we’ve pointed out before here in Money Morning, the big question is whether AnteoTech’s products will make it to market in time.
First-mover market advantage will be crucial in this space and with hundreds of products already launched, it is uncertain right now if AnteoTech will consolidate enough market presence.
That said, even if AnteoTech’s ART product doesn’t capture sufficient market share, acquiring WHO and CE mark approval can refine the company’s track record and show future customers that AnteoTech is a skilled nanotechnology company.
This may make it easier to market and sell ADO’s other products.
If smaller-cap stocks like AnteoTech interest you but you’re unsure how to find ones with great potential, then I think you will enjoy reading this free report on four high-value small-caps.
Small-cap investment is all about information advantage, so I highly recommend reading through the report.
Regards,
Lachlann Tierney
For Money Morning


Comments