The MGC Pharmaceuticals Ltd [ASX:MXC] announced that its distribution partner placed an order for 1,000 units of MXC’s CimetrA on an expedited basis.
The units were ordered to fast track the approval process for the medication to be distributed and sold in the US.
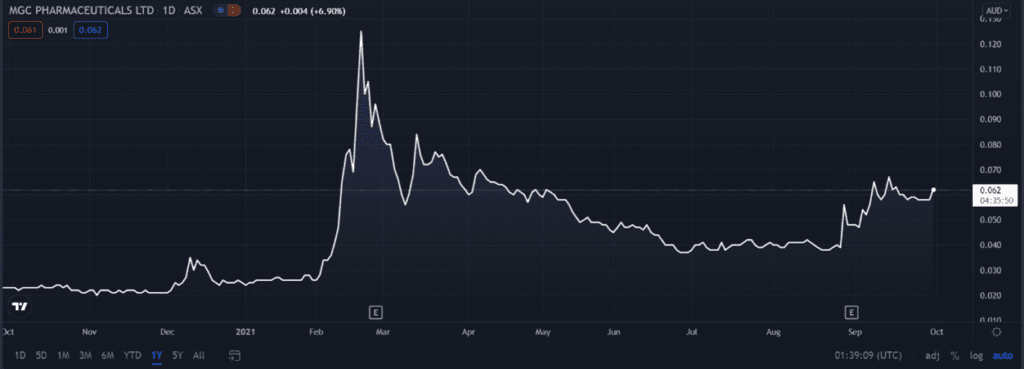
The expedited order buoyed the market. MGC Pharmaceuticals Ltd [ASX:MXC] share price is currently up 6.90%.
Let’s look at today’s announcement in detail and see what can possibly be next for MXC shares.
AMC Holdings orders 1,000 units of CimetrA
AMC — MGC Pharma’s distribution partner — placed an initial order for 1,000 units of CimetrA.
CimetrA is the pharmaceutical version of MXC’s nutraceutical-branded product, ArtemiC.
MXC says CimetrA is anti-inflammatory and can reduce the hyper-inflammatory response seen in some COVID-19 patients, known as ‘cytokine storm’.
The units will be shipped to a Tampa area pharmacy where they will be analysed.
It marks the first step in undertaking a material commercial order of MGC Pharma products as discussed under the US Supply Agreement signed by both the parties in August 2021.
Both MXC and AMC are also working to formalise their business and research partnership and are ‘confident they remain on track to do so.’
Discover our top three ASX-listed pot stocks in 2021. Click here to learn more.
Additionally, AMC is currently working with the University of Florida in Tampa and the Holy Cross Hospital in Fort Lauderdale to submit CimetrA and CogniCann to their respective Internal Review Boards for approval.
Brent Yessin, General Counsel for AMC, made his case for a viable COVID-19 treatment product:
‘As the US endures a COVID spike, keeping COVID positive patients out of the hospital has become a major policy objective of countless state Governors and their health departments.
‘For every 1000 patients who are treated effectively and kept out of hospital, the state or healthcare systems save US$40M, a rare instance where clinical and financial imperatives are perfectly harmonized.’
What’s next for the MXC Share Price?
Today’s update follows MXC’s CannEpil+ product to treat refractory epilepsy, gaining approval for UK import.
MGC Pharma was also recently granted a permit to import its proposed COVID-19 treatment to India for emergency use drug registration.
Bottom line is that MXC is moving quickly on multiple fronts.
What’s next for the bio-pharma stock?
US-based clinical trials are being established for MGC Pharma’s phytomedicine products in Florida, Texas, the NYC area, and Kentucky and are expected to commence in Q4 2021.
Meaning investors can expect more developments from the stock in the near term.
MGC Pharma CEO and Managing Director Roby Zomer said:
‘AMC are already making significant progress in propelling CimetrA, CannEpil and CogniCann® to Clinical Trial stage in the US and we are pleased that they have cemented relationships with these prestigious institutions for the continued research into our products.’
Now, if you are interested in more small-cap stocks with exciting potential, I suggest checking out this report by our small-cap analyst Ryan Clarkson-Ledward.
The report takes you through four companies Ryan believes are small-cap gems.
Regards,
Kiryll Prakapenka,
For Money Morning
PS: Our publication Money Morning is a fantastic place to start on your investment journey. We talk about the big trends driving the most innovative stocks on the ASX. Learn all about it here