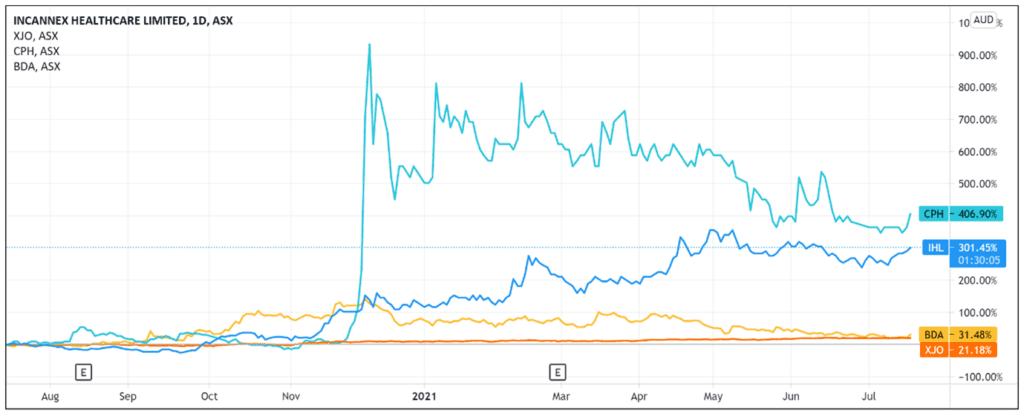
The Incannex Healthcare Ltd [ASX:IHL] share price rose 5.5% in early trade after IHL engaged a firm to manufacture IHL-675A soft-gel capsules pending clinical trials.
The development of IHL’s anti-inflammatory multiuse drug product — IHL-675A — is progressing after Incannex hired Procaps to develop and manufacture soft-gel capsules using Procaps’ patented Unigel technology.
Shares in the pharmaceutical development company are up 300% over the last 12 months.
IHL-675A update
Incannex engaged Procaps to provide an end-to-end service.
This will include formulation development, clinical trial supply, and commercial manufacture if clinical trials are successful.
Incannex said the partnership with Procaps would grant it access to a range of proprietary technologies, including Procaps’ patented Unigel apparatus that encases solid dosages within capsules.
What is IHL-675A?
IHL-675A is Icannex’s proposed medical product containing hydroxychloroquine and cannabidiol.
Incannex recently expanded its development program to assess its potential to help with lung inflammation, rheumatoid arthritis, and inflammatory bowel disease.
PS: We reveal four little-known small-cap stocks that cannot be ignored…Download your free report now.
IHL Share Price ASX Outlook
As an end-to-end engagement, Incannex has also tasked Procaps with the commercial manufacture of IHL-675A if clinical trials succeed.
This may give investors a glimpse at IHL’s logistics strategy once its products are ready for commercial distribution.
As IHL CEO Joel Latham today commented:
‘Procaps offers Incannex a complete supply chain solution for a sophisticated, GMP-grade product.
‘Manufacturing at Procaps will support our clinical trial programs and can also quickly ramp up production for commercial supply upon successful clinical trial outcomes.’
That said, today’s update did not disclose the financial details of IHL’s engagement with Procaps nor the financial arrangements entailed in Procaps commercially supplying IHL-675A.
Of course, financial arrangements regarding commercial rollout were likely not discussed because Incannex has many hurdles left before contemplating commercial production.
The commercial supply of IHL-675A depends first and foremost on clinical success. And IHL-675A is still making its way through Phase 1 clinical trials.
If this phase succeeds, it will trigger three FDA investigational new drug applications. If these applications are approved, then IHL-675A will undergo Phase 2 and Phase 3 clinical trials.
No doubt, the market will track the product’s progress closely.
Incannex, like other ASX pot stocks, has certainly caught the eyes of investors lately who are seeking growth markets.
If you are interested in pot stocks and wish to research the growing CBD healthcare industry and what investment opportunities are out there, this free report is highly recommended.
Regards,
Lachlann Tierney,
For Money Morning
PS: Our publication Money Morning is a fantastic place to start on your investment journey. We talk about the big trends driving the most innovative stocks on the ASX. Learn all about it here