Immunotherapies and cancer-fighting research group Imugene [ASX:IMU] has announced its Phase One MAST (metastatic advanced solid tumours) trial and has now moved through to the next stage.
The announcement comes just days after IMU said it had dosed its first patient with the PD1-Vaxx for non-small cell lung cancer (NSCLC) combination trials.
Investors were supporting the headway made by the biotech group, with the stock receiving an upvoting of 7.5% by the early afternoon on Friday.
IMU was trading for 10 cents a share at the time of writing, the share still heavily discounted after a 13% slide in the month, 31% in the year so far and trending at 35% below the S&P:
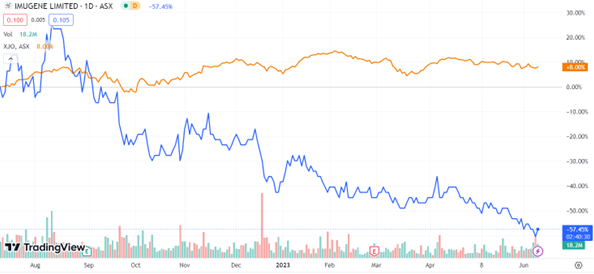
Source: tradingview.com
Imugene advances cancer-fighting tactics with MAST trial
Reporting from Sydney, the immune-oncology producer announced the next phase of testing for its cancer-targeting virus is ready to move on to the next cohort of patients.
Phase One of MAST came into play to evaluate the safety of its novel cancer-killing virus CF33-hNIS (VAXINIA). It has progressed to the next cohort of the intravenous (IV) arms of both the monotherapy and combination study.
The virus, created by the City of Hope, has been developed to shrink colon, lung, breast, ovarian, and pancreatic cancer tumours in labs and animal models.
IMU hopes to recruit up to 100 patients across sites in the US and Australia to test the virus, and with trials having commenced in May last year, cohort three is now complete, opening up cohort four.
The MAST trial is expected to run for around 24 months and is being funded from existing budgets and resources.
The next round of tests will examine monotherapy and combination studies with new patients.
Imugene also revealed that cohort one of the IV arm of the combination study with Pembrolizumab has been cleared, allowing cohort two to begin.
Imugene’s managing director and CEO, Leslie Chong, said:
‘As we continue to move through the cohorts at pace, we’re aiming to have this high-quality science peer reviewed and recognised within publications or conferences befitting of its results and potential benefit to patients in need.’
Last week Imugene announced that the first combination patient was dosed with PD1-Vaxx and the Immune Checkpoint Inhibitor for its lung cancer clinical trial.
This was a combination cohort of the Imprinter study, a clinical trial to evaluate the PD1- Vaxx, a B-cell activating immunotherapy to work in combination with atezolizumab (Tecentriq), an immune checkpoint inhibitor targeting PD-L1 from Roche, in patients with NSCLC.
Tecentriq was the first approved cancer immunotherapy for supporting NSCLC and the first approved cancer immunotherapy for front-line treatment of adults with extensive-stage small cell lung cancer (SCLC) in combination treatments.
Ms Chong commented:
‘PD1-Vaxx has shown a tolerable safety profile and encouraging efficacy in patients with NSCLC, and we are looking forward to evaluating PD1-Vaxx with atezolizumab in ICI treatment-naïve and pre-treated NSCLC patients.’
From biotech to Tik-Stocks
Did you know that previous no-name and inconspicuous small-cap Stemcell United [ASX:SCU] catapulted 8,284% in two days on a medicinal cannabis venture?
It wasn’t so insignificant then, and it happened so suddenly.
Cann Group [ASX:CAN] also jumped 30 cents to $4 in a matter of months on another viral explosion.
If you know where the next burst of mania is going to be, you can really take advantage of the hype.
This is why our experts bring you Tik-Stocks, a sort of cousin to ‘meme stocks’ — you know, those stocks that go through the roof based on social media coverage.
These stocks can become overvalued in the blink of an eye. This means you need to time them right.
Intrigued? Click here for more.
Regards,
Mahlia Stewart,
For Money Morning

