The share price of oncology-focused biotech firm, KAZIA Therapeutics Ltd [ASX:KZA], soared this morning on the receipt of FDA designation for its brain cancer treatment.
KZA’s therapeutic, known as paxalisib, entered a phase II clinical trial in 2018.
Analysis of this trial was presented last month, demonstrating promising results when compared to the current standard of care.
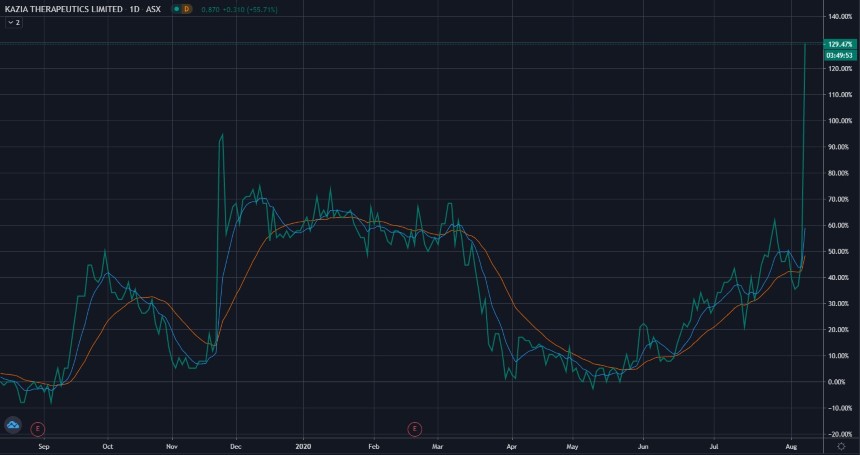
At time of writing the KZA share price is up 26 cents or 46.43%, trading at 82 cents per share.
KZA’s shares have broken their 52-week high of 80 cents and are trading around a level not seen since 2018.
Source: Tradingview
KAZIA to treat childhood cancer too
KZA’s announcement this morning relates to the FDA’s decision to award the company Rare Paediatric Disease Designation (RPDD).
The RPDD is for the treatment of Diffuse Intrinsic Pontine Glioma (DIPG), a rare and highly aggressive childhood brain cancer.
The designation does not mean the treatment has been approved by the FDA.
Four Innovative Aussie Stocks That Could Shoot Up after Lockdown
Rather, KZA has the opportunity to seek a shortened FDA review period for any of its drugs.
The shortened period would mean reviews are cut from 12 to six months.
According to KZA, the FDA’s RPDD program is intended to advance the development of treatments for certain life-threatening diseases by providing incentives to industry.
These incentives are called rare paediatric disease priority review vouchers (PRV).
PRVs can be sold to other companies and have historically fetched prices in the tens to hundreds of millions of dollars.
For patients diagnosed with DIPG, there are currently no FDA-approved drug treatments, and the average survival from diagnosis is around 9.5 months.
Though the company said glioblastoma remains their primary focus for paxalisib, positive clinical data from DIPG studies could boost their chances of obtaining a PRV.
Meaning KZA will be able to significantly cut review periods for their drugs.
Is KAZIA a gamble?
Life-saving potential aside, KZA presents and interesting profile.
As I mentioned earlier, KZA have has some success with paxalisib already.
This was with treating glioblastoma, the most common and aggressive form of primary brain cancer.
KZA may be seeking a shorter path to obtaining a PRV by attempting to treat a rare brain cancer with no approved treatments.
It’s a double-edged sword.
If clinical trials are a success, then KZA could get their other treatments through FDA approval.
Or they could sell the highly valuable PRV to another drug company.
In 2019, five paediatric PRVs were granted by FDA.
So keep that in mind.
On the other hand, the clinical trial may show no significant results, likely hurting KZA’s chances for DIPG approval.
Recruitment for phase 1 clinical trial has been completed and initial data is expected before the end of the year.
Kind regards,
Lachlann Tierney
Money Morning
PS: Four Well-Positioned Small-Cap Stocks: These innovative Aussie companies are well placed to capitalise on post-lockdown megatrends. Click here to learn more.